Cold Chain for Beginners
What is the Cold Chain?
The cold chain is a temperature-controlled supply chain system crucial for industries such as pharmaceuticals, biotechnology, and food. Its primary purpose is to ensure that sensitive products—such as vaccines, drugs, and biological materials—maintain their safety and effectiveness by staying within prescribed temperature ranges from manufacture to end use.


Why is Cold Chain Important?
For products like vaccines and biologics, even brief temperature excursions outside recommended storage ranges can jeopardize their safety and efficacy. The COVID-19 vaccine rollouts highlighted the cold chain’s vital role, making it a global focus for logistics, healthcare, and life sciences.
Cold Chain Terminology
No power source, uses insulation and refrigerants (e.g., gel packs, phase change materials) to maintain product temperature.
Uses powered heating or cooling mechanisms to precisely control internal temperature.
Cooling agent materials such as gel packs, bricks, or bottles to keep products at the right temperature.
Substances that absorb or release energy as they change from solid to liquid or vice versa, helping regulate internal temperatures.
Stabilizing materials at a target temperature before shipment.
Acclimating refrigerants to a specific temperature just before packing.
Adjusting packaging or conditioning depending on the season (e.g., summer vs. winter).
Universal Pack-out: Standardized packaging that works year-round, regardless of season.
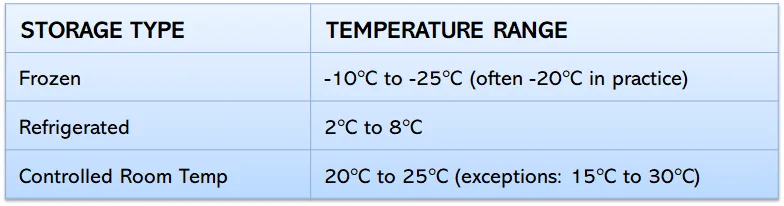
Typical Temperature Requirements
Maintaining the correct storage temperature is essential for preserving the quality and effectiveness of pharmaceutical products. Each type of medication has specific temperature requirements to ensure its safety and potency throughout its shelf life and distribution journey. The following storage guidelines outline the common temperature ranges needed for different categories of pharmaceutical products.
Key Considerations for Cold Chain Packaging
Mode of Transport
Truck, plane, boat, or drone.
Origin and Destination
These impact shipping time and temperature issues.
Duration of Transit
The total shipment time impacts the type of solution required.
Expected Ambient Temperatures
Exposure profiles change based on region and season.
Handling
The amount of handling, loading, and unloading can introduce temperature risks.
Evaluating Packaging Performance
To ensure compliance and product protection, evaluate packaging using laboratory tests that simulate real-world temperature challenges. Industry standards such as PDA Technical Report 39 and ASTM procedures guide these evaluations, involving:
- Triplicate testing under worst-case and best-case conditions
- Simulations of both summer and winter temperature profiles
- Testing with both maximum and minimum load scenarios
- Performance Qualifications (PQ) and monitoring technologies should be used throughout the shipping process to validate ongoing cold chain effectiveness.


Best Practices for Cold Chain Management
Effective cold chain management ensures product quality, safety, and efficiency from origin to destination. By following proven best practices, organizations can strengthen reliability, reduce waste, and maintain compliance throughout the supply chain.
- Collaborate with key stakeholders to collect data and make informed decisions.
- Regularly review and update processes for continuous improvement.
- Apply proactive temperature monitoring to ensure compliance and quickly address issues.
Have questions about choosing a cold chain solution? Contact our team for tailored advice and solutions for your specific needs.
